Case Study: Our first Track&Trace prototype

We are currently working on a project where Vega IT and its partner, Assistive Technology, have joined forces in order to create a prototype demonstrator of a medical device Track & Trace system. The idea behind it is to think ahead for our partner while also doing the preparation work for them. By treating the project as our own investment in terms of the work and preparation being put in, we believe it is safe to say that its applicability is assured.
The reason for this certainty is the research we have done on the new medical device regulations soon to influence the certification of all new medical devices. We have experience in terms of how companies plan for upcoming regulations (the Track and Trace for Pharma) and we see this happening again.
Also, I will try to introduce you to the rest of our Innovation Pool team and I will try to explain what is it exactly that we are doing. The benefits for the team are planning ahead and working on new demonstrators as preparation for those future projects when and if the partner shows interest in.
Innovation Pool Research & Development team
My colleagues participating in this project are Ivana Tešanović, Nenad Rad, Vuk Raković, Velibor Stančić and myself - Goran Todorović.
Before we even began with prototype development - and I will get back to this a bit later -, for the first couple of months we were focused on research. The reason why we focused on this at the first stage was the specificity of the project.
This is a unique type of project, being a small startup that is a part of Vega IT. The usual scenario is that we have clients who approach us with a clear vision of what they are aiming for, meaning they have precise specifications and requirements.
With the possibility to create our own requirements and implement functionalities in terms of our research, we created an imperative of making it likeable for our potential clients. The driving force are also the new regulations - not fully specified since even the EU tools are not yet available (EUDAMED) - using FDA as guidance of what will probably come and leaving ourselves to design a generic system that can be changed as specifications become available.
Let's get back to our prototype
I am assuming that a large percentage of us have had a chance to order something via eBay or Aliexpress. And this is exactly one of the examples that I am going to use so you can easily understand what our Track and Trace system does.
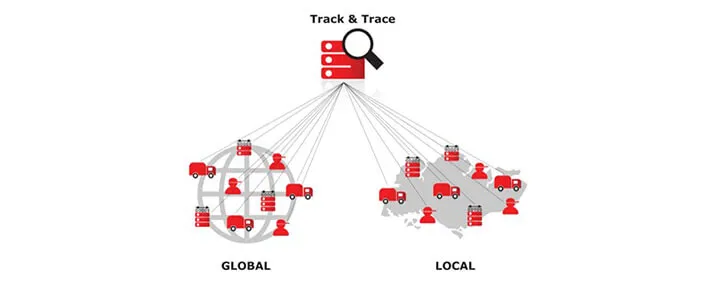
So let's begin with this scenario. You saw something really cool on eBay, you’ve ordered it and now you are impatiently waiting for the package to arrive. You have a tracking number that, once typed into a website, gives you the exact information about your package - where it is now, which city, what is the order destination, along with all the accompanying dates.
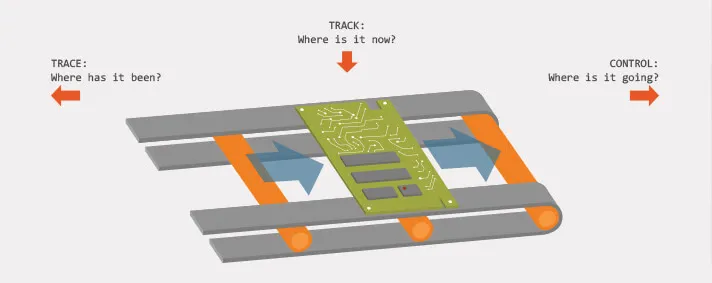
What is Track and what is Trace?
A common definition of Track and Trace in distribution and logistics of different types of goods would be that it represents the process of determining the current and past location and any other relevant information about our unique order. Lets separate those two terms a bit more. Track represents information about the present state or location of some device (for example), while Trace represents the entire location history of the device.
Track and trace inside factories
We can use the same principle, mentioned above, for factories and other production facilities where we have products on conveyor belts which are further going to be packed. A tracking system allows us to know, in any given moment, where exactly our device is (inside the factory). From the moment the product leaves the factory we lose track of it. This can become a difficulty because we are not sure anymore in which warehouse it is, etc.
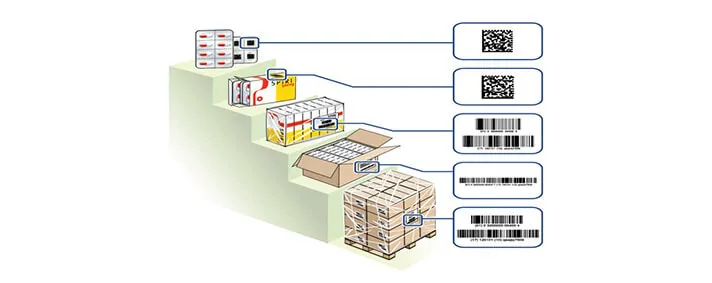
The situation is pretty much the same for medical devices. Currently, there is no Track and Trace system for those kind of devices which are produced inside EU.
The EU accepted a wide range of regulations delivering a large number of rules and standards that all medical device distributors need to comply with. The transition period for companies to adopt those regulations is a timeline from 2018 to 2022. Those regulations sparked our motivation to create the prototype. The fact that those regulations are directly related to Track & Trace outside the factories makes it all much easier.
What our prototype does is it demonstrate to clients, or producers, that this system will be available even before the transition happens in the EU. Our principle is - the regulations are adopted and the next thing we have available is something to offer without hesitation. Basically, we will be the first ones to offer something like this to the market.
Our prototype
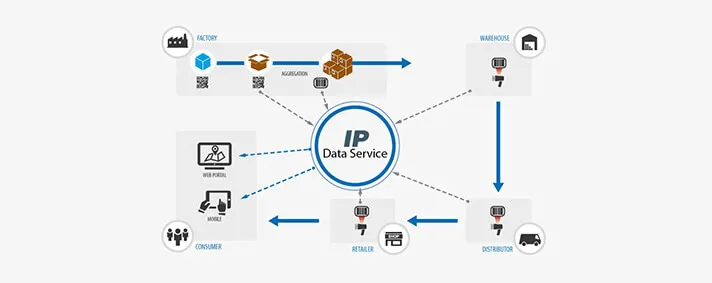
As I already mentioned, we are spreading above the domains of factories and we offer the complete Track & Trace devices for the end user. The user can be an individual or a medical institution. Once the device leaves the factory and goes to the warehouse we are going to be able to determine the exact place where it is coming from. It would be enough for someone to scan the unique barcode on the device’s sticker and the information about that particular device will be available to them.
The same principle applies to distributors and sellers. The end users can benefit largely, as well. It would be possible for them to, by using a certain web portal or mobile device, scan the barcode and get the desired info about the device.
This is just one in a myriad of characteristics making our prototype stand out next to the other available solutions. A Track & Trace system like ours is very useful when it comes to a situation of withdrawing a device from the market due to a malfunction.
Up until now this was a hard and long process, since there was no insight into the institutions where a particular device was used nor was any other information acquired outside of production available. In certain situations this can become a risk for people. This issue was precisely the reason for the implementation of new regulations. Let us take an example of the scandal that triggered the revision of the regulations (PIP breast) implants - an industrial grade silicon was used in manufacturing as a cheaper ingredient, instead of using medical grade materials, ie no traceability for the authorities/patients.
The other advantage of our solution is the potential it has to prevent copying and plagiarism. The end user has no insight into the device’s history and therefore doesn’t know whether or not the device is valid. Our solution would enable for the end user to take and scan the barcode on any device by a supported manufacturer via a mobile phone or by inputting the data onto a web portal in order to get all the device’s detailed information.
Another important fact that must be taken into account is that there are certain medical devices that can be assembled outside the factory. So, for example, a multiple components device can be assembled in a warehouse. An MRI machine has many parts that can be produced by different manufacturers and our solution will enable insight into all of the device’s components. This means that in case of a malfunction and identification of the malfunctioning component, we would make it possible to know which part is broken and what component was it replaced with. The core of our prototype is the Data Service through which all takes place.This is an assembly of a number of distributing agents that make it all possible. What makes our Data Service stand out is the fact that it will enable communication with the public database system suggested by the new regulations.
On this image we have an example of a sticker for an implant, that is, a medical device. This is an example of a sticker that follows the new regulations adopted by the EU. There we can see sufficient data about the device, and when I was talking about “taking and scanning the device” I was referring to scanning the barcode on this sticker in order to gather even more information about the device, its origin, etc.
Our MVP
Our prototype is just in its first phase of development. We are steadily approaching our initial version of a minimal demonstrator of technology to attract interest in a full proposal which will be ready for demonstration. While we are waiting for this to happen we would like to hear more from you and your thoughts about Track and Trace systems.

